Screen and treat with HPV testing in LMIC countries
Quote this article as:
U Rani Poli & P Petignat (2017). Screen and treat with HPV testing in LMIC countries. www.HPVWorld.com, 9
Around 85% of cervical cancer deaths occur in low and medium-income countries (LMIC) essentially because of the lack of screening. Cytology-based cervical cancer screening programs have successfully reduced cervical incidence in high-income countries, but not in LMIC, essentially because of high-cost and logistic limitations. A “screen-and-treat” approach for cervical prevention is based on a screening test result followed by treatment in the same visit. This strategy allows reducing travel time, minimizing the number of visits, transport, childcare needs and reducing the cost. It has been demonstrated in the LMIC context that adding a delay between screening, obtaining results and proposing treatment induces a loss of follow-up.
Therefore, an approach incorporating diagnosis procedure followed by an immediate management should be prioritizing to optimize cervical cancer screening program in LMIC. The visual inspection with acetic acid (VIA) or Lugol’s iodine (VILI) testing as an alternative to cytology has the advantage of being low-cost, easy to be carried out by nurses of midwifes after short training and offer the option of treatment immediately or shortly after diagnostic testing. Limitation of VIA-based screening is that it is health care provider-dependent and lacks reliable assurance control. These limitations have favored the search of alternative approach for LMIC like HPV testing. This method should add objectivity and performance by detecting cases of neoplasia that visual approach has failed to detect and offer the possibility that sample collection can be performed by the patient herself, not requiring trained personnel and infrastructure to perform a pelvic examination, and thus increasing acceptability and screening coverage.
Screening campaign in Rasulpura, Hyderabad.
A randomized trial conducted in South Africa having adopted a HPV–based approach, demonstrated that it is a highly effective strategy with a significant reduction of CIN2+ lesion. This approach is far more accurate, sensitive and robust than VIA in detecting cervical intraepithelial neoplasia grade 2 and worse (CIN2+). After 36 months of follow-up, the cumulative risk of CIN2+ was reduced by 73% in HPV-negative women and 32% in VIA-negative women as compared to the control (untreated) arm1 Other report demonstrated that HPV testing strategy conducted in LMIC is associated with decreased cervical cancer-related mortality2.
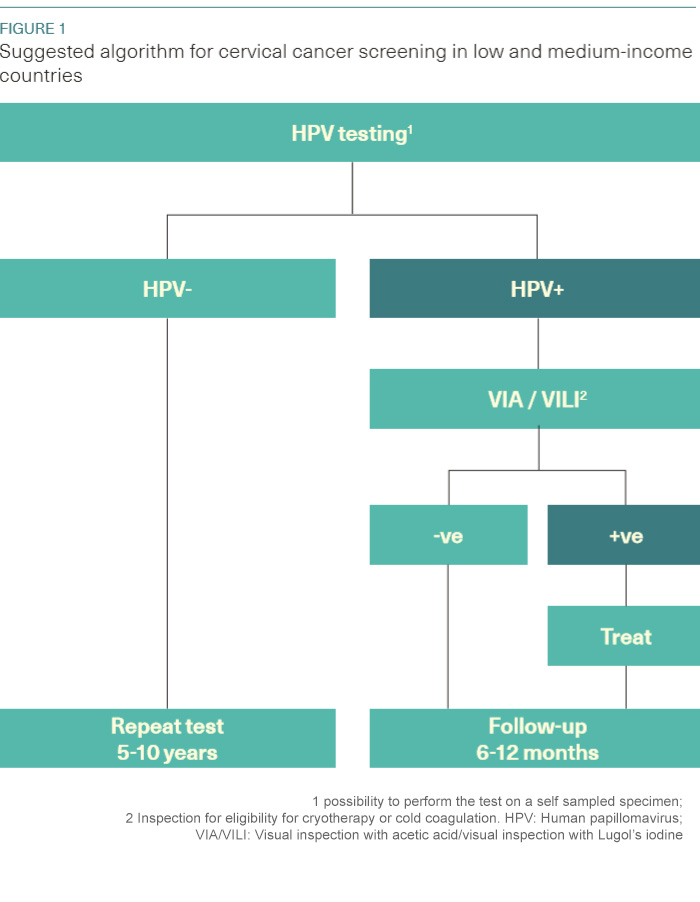
HPV-based screening is recommended by WHO as an alternative for cervical cancer screening (WHO 2014). Until recently, the greatest limitations of HPV testing were the need for expensive laboratory infrastructure and the 4–7 h time to process the test. The development of rapid molecular methods for detecting HPV DNA (e.g., care HPV® - Qiagen, GeneXpert® - Cepheid) for screening or other point-of-care type of tests make a “screen and treat” strategy more feasible. Limitation of HPV-based screening is its low positive predictive value, since it does not directly test for cancer, but for HPV infection. This indicates that a triage test following a positive HPV results may be necessary to limit the rate of false positive and consequently reduce the harm of overtreatment. Integrating HPV screening with a VIA triage test may offer the dual benefits of HPV screening to maximize the detection and VIA for treatment triage of HPV-positive women (Figure 1).
The START UP study conducted in Hyderabad, India has evaluated the careHPV test as primary screening test (HPV self-sampling)3. HPV-positive women were triaged with VIA, and VIA-positive women were treated by cryotherapy. Results support that VIA used as a triage test for HPV-positive women can reduce the number of overtreatments, with 19% of HPV-positive being treated. However, VIA as a triage test missed 40% of CIN2+ lesions but performed similar to colposcopy in detection of CIN3 and invasive cancers in this study. Hence VIA can be used for treatment triage. Similar results have been reported in a study conducted in sub-Saharan Africa using VIA/ VILI as triage test for HPV-positive women with an extremely low sensitivity (25%) for CIN2+ detection4.There is probably room for VIA improvement that still need to be investigated like the use of cervicography to assure quality assurance of the method.
In conclusion, new laboratory-independent and affordable HPV tests are available, providing immediate results and making it possible to screen and treat women during the same visit. Adding VIA to HPV primary testing may be a well-suited method for LMIC and provides an unprecedented opportunity to develop cervical screening programs with a single-visit approach. However, questions are still open as how such a test could be introduced in an effective manner in LMIC.
References
1. Denny L et al., Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial.J Natl Cancer Inst. 2010 Oct 20;102(20):1557-67.
2. Sankaranarayanan R, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360 : 1385-94.
3. Jeronimo J, et al., START-UP Study Group. A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. Int J Gynecol Cancer 2014;24:576–85.
4. Bigoni J1, et al., Petignat P. Cervical cancer screening in sub-SaharanAfrica: a randomized trial of VIA versus cytology for triage of HPV-positive women. Int J Cancer. 2015 Jul 1;137(1):127-34.