Primary hrHPV population screening for cervical cancer in the Netherlands
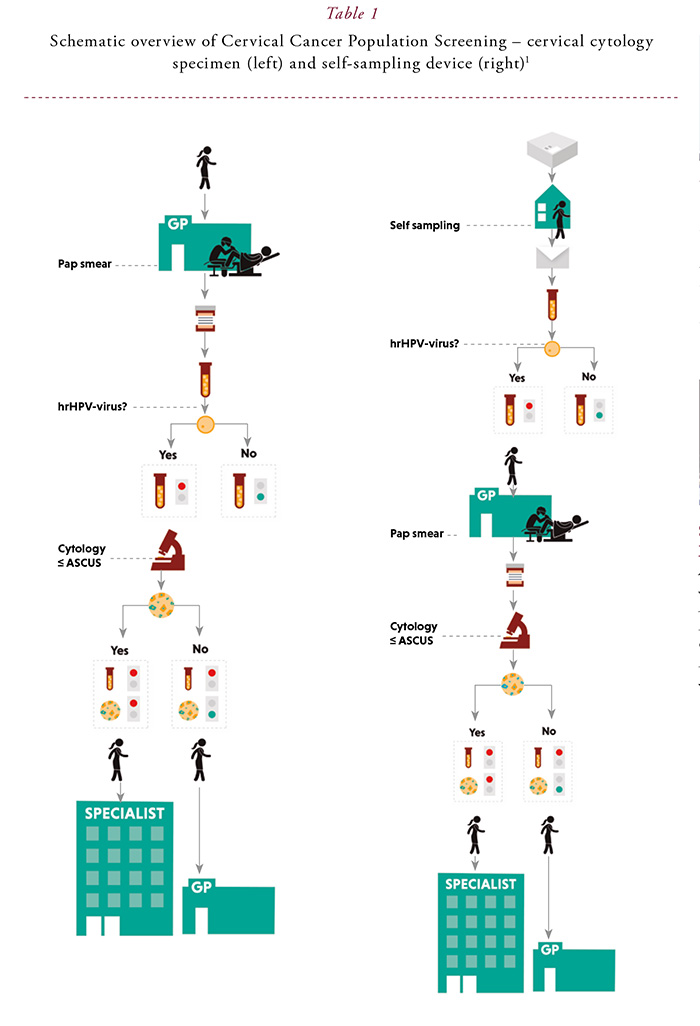
In 2017, the Dutch cervical cancer screening programme started the implementation of primary hrHPV screening with cytology triage at the general practitioner (GP) office. All hrHPV-positive women with cytological abnormalities (≥ ASCUS) are referred to the gynaecologist, instead of referring only women with HSIL or worse in the previous cytology-based screening programme. If there are no abnormal cells, the woman is advised to have a second cytology test in six months as part of the screening programme.
Liquid-based cervical cytology specimens are taken at the GP office but women who do not respond to the initial invitation can order a self-sampling device. In case of a hrHPV-positive self-sample, a GP-visit is needed to collect a cervical cytology specimen, because cytology is not possible on self-samples. Figure 1 presents a schematic overview of the screening programme.
Invitation scheme
Women aged 30, 35, 40, 50 or 60 years old receive an invitation for the population screening. At the age of 45 and 55 years, only women who had a hrHPV test positive or not performed at ages 40 or 50 years, respectively, receive an invitation. Women who had a hrHPV-positive test at the age of 60 years, receive a final invitation at the age of 65.
Tests performed
All laboratories use the cobas® 4800 HPV test (Roche Diagnostics, Alameda CA, USA) to test the clinical and self-samples. The cobas® 4800 HPV test is a CE/IVD certified kit for use in combination with the cobas® 4800 nucleic acid extraction PCR setup, real-time amplification and software system. As part of the assay procedure, each sample is tested for the presence of human cells by amplification of the human beta-globin gene.
The Evalyn® Brush (Rovers Medical Devices, Oss, the Netherlands) is used for self-sampling whereas ThinPrep® (Hologic, Bedford, MA, USA) is used as transport medium for cervical cytology specimens.
Quality control in general
The primary hrHPV screening programme implementation reduced the number of laboratories from 40 to 5. Two national reference officers, one for HPV and one for cytology, chair the national quality platform with representatives of the five laboratories. This platform exchanges experiences and methods used to enhance a uniform practical approach of the screening. The five laboratories are accredited by the Dutch CCKL (Coordination Commission to promote the Quality Control of Laboratory Research) or ISO 15189 and back up for each other if needed.
As part of the preparations towards the renewed programme, the suppliers of the HPV test and thin-layer cytology trained the employees of the laboratories. Additionally, cytologists and pathologists analysed two learning sets of samples to get used to a higher percentage of cytological abnormalities. Furthermore, there is a quality control programme on HPV and cytology on a structural basis.
Quality control of HPV and cytology testing
Besides proficiency panels of hrHPV samples and cytological samples, the quality control programme includes monitoring of the analytical performance of the hrHPV-test:
- A verification and release programme for acceptance testing of equipment upon installation, repair or major maintenance activity. This programme is also used to test and release (new lots) of critical reagents
- A run control programme with a manufacturer-independent control sample in each HPV run. In addition, standardized procedures and protocols are written by the quality platform and used by all laboratories.
The results of the first year of primary hrHPV screening will be published on the English website of the National Institute for Public Health and the Environment as soon as available.2

References
1. National Institute for Public Health and the Environment. 2017. Framework for the Execution of Cervical Cancer Population Screening. [Available from: Framework_for_the_Execution_of_Cervical_Cancer_Population_Screening].
2. National Institute for Public Health and the Environment. 2018. Cervical cancer screening programme. [Available from: https://www.rivm.nl/en/Topics/C/Cervical_cancer_ screening_programme].



