The Biological Rationale For A One Dose HPV Vaccine
Although the antigens in the HPV vaccines are designated “virus-like particles” (VLPs) because they mimic the outer shell of authentic HPV virions, they are generally considered to be a type of subunit vaccine in that they are composed of a single highly purified protein, in this case the L1 major capsid protein, and are entirely noninfectious.
All licensed subunit vaccines are administered in multi-dose prime/ boost regimens. It was therefore surprising when post-hoc dose-stratified analyses from the bivalent and quadrivalent HPV vaccines efficacy trials suggested non-inferior protection from incident infection by vaccine-targeted HPV types in young women who received only a single priming dose compared to the per protocol three-dose series [1,2].
Although there was no indication that risk of HPV acquisition differed by number of vaccine doses received, the fact that the women were not randomized to receive less than three doses raises the possibility that differences in HPV exposure or other factors in the dose groups could bias the results. Before embarking on a large trial to rigorously evaluate the efficacy of less than three doses of the HPV vaccines, it therefore seems prudent to consider if it is biologically plausible that the HPV vaccines could be effective after a single dose, whereas other subunit vaccines are not. It is reassuring that consideration of both immunologic and virologic factors support the exceptional efficacy of HPV vaccines, even after a single dose.
It was therefore surprising when post-hoc dose-stratified analyses from the bivalent and quadrivalent HPV vaccines efficacy trials suggested non-inferior protection from incident infection by vaccine-targeted HPV types in young women who received only a single priming dose compared to the per protocol three-dose series
Immunologic Factors.
The most compelling support for the conjecture that HPV vaccines will induce long term protection after a single dose are the findings that the vaccines induce strong and durable neutralizing antibody responses against the targeted types in essentially all one dose recipients.
For the bivalent HPV vaccine, the geometric mean of antibody titers (GMT) after four years were only four-fold lower in one dose compared to three-dose recipients [3] (Figure 1), and this ratio has been maintained out to seven years [4]. For the quadrivalent HPV vaccine, similar differences between the antibody levels in the one- and three-dose recipients were reported in a three-year interim analysis. Direct comparison between the levels induced by these vaccines among one-dose recipients is currently not possible because different assays were used to quantify the antibody responses. The exceptional immunogenicity of HPV vaccines can largely be attributed to the structure of the HPV vaccine antigen. Typical subunit vaccines are composed of monomeric or low valence multimeric proteins or carbohydrate/protein conjugates. In contrast, HPV VLPs are composed of 360 ordered protein subunits that form a particulate 55nm structure displaying a repetitive array of epitopes on their surface. The engagement of these repetitive elements by the B-cell receptors (BCRs) on naïve B-cells is believed to transmit exceptionally strong activation and survival signals to the cells leading to consistent induction of memory B-cells, and, most importantly, long lived plasma cells (LLPCs) that continuously produce antibodies for many years [5] (Figure 2).
Epitope spacing of 50-100Å appears to be critical. This spacing is commonly found on microbial surfaces but not on body surfaces normally exposed to the systemic immune system. Th e particulate and repetitive structure of VLPs likely contributes to B-cell immunity in several additional ways. Particles of this size effi ciently traffi c to lymph nodes and are also effi ciently phagocytized by antigen presenting cells for the initiation of immune responses and the generation of cognate T helper cells. Th e poly-valence of VLPs also leads to stable binding of nature low avidity IgM and complement, promoting their acquisition by follicular dendritic cells, which are key components in inducing humoral immune responses in the lymph node. Note that the hepatitis B vaccine is also considered a VLP but appears to be much less immunogenic after a single dose, perhaps because it has a fewer subunits and/or because they fl oat in a lipid bilayer.
To summarize, by mimicking the key structural features of authentic viruses, the HPV VLPs consistently induce potent and long lasting humoral responses that more closely resemble the anti-virion responses to an acute virus infection or a single dose of a live-attenuated virus vaccine than the response to simple subunit vaccines.
By mimicking the key structural features of authentic viruses, the HPV VLPs consistently induce potent and long lasting humoral responses
Virologic Factors.
Several virologic factors also likely contribute to the exceptional effi cacy of the HPV vaccines. First, Papillomaviruses (PVs) have an unusual life cycle that is entirely confi ned to a stratifi ed squamous epithelium. By producing their virions in the superfi cial layers and shedding them from the epithelial surface, the viruses minimize exposure of these highly immunogenic structures to the systemic immune system, and thereby can persistently produce infectious virions that are not subject to inactivation by neutralizing antibodies. Overall, PVs have evolved to maintain immune ignorance rather than evolving mechanisms to actively counteract systemic immunity. Th is mechanism of immune escape can be easily overcome by parenteral injection of the VLPs.
Second, studies in animal models have found that the mechanism that the viruses use to infect their target tissues make them exceptionally susceptible to vaccine-induced virion neutralizing antibodies [6]. To initiate infection, the virions must bind to specifi cally modifi ed forms of heparan sulfate restricted to the basement membrane in normal tissue (Figure 1). Exposure of the basement membrane requires epithelial disruption. Direct exudation of systemic antibodies occurs at these sites, such that the virions are subject to an increasing gradient of antibody concentration as they approach their binding site. Basement membrane binding initiates a series of conformational changes that are required for exposure of the keratinocyte receptor-binding site on the virion surface (Figure 3). Importantly, this process and the subsequent process of virion internalization by the basal keratinocyte take several hours. The slow kinetics of infection, much slower than for other well-characterized viruses, provides an exceptional length of time for vaccine-induced antibodies to disrupt the process. Inactivation can occur even after basement membrane binding, perhaps due to Fc receptor-mediated phagocytosis of the virion/antibody complex. Note that neutrophils and macrophages are specifi cally attracted to sites of epithelial disruption.
The slow kinetics of infection, much slower than for other well-characterized viruses, provides an exceptional length of time for vaccine-induced antibodies to disrupt the process.
Experiments in mice involving passive transfer of sera from VLP vaccine individuals into naïve recipients indicate that levels of circulating antibodies that are 100-fold lower than the minimum detected in in vitro assays are suffi cient to protect from high-dose cervicovaginal challenge in vivo, implying that there are potent mechanisms of antibody-mediated infection inhibition that are not measured in vitro [7]. In light of these observations, it is not surprising that the four-fold lower longterm antibody titers in one-dose recipients did not diminish the apparent protective efficacy of the HPV vaccines.
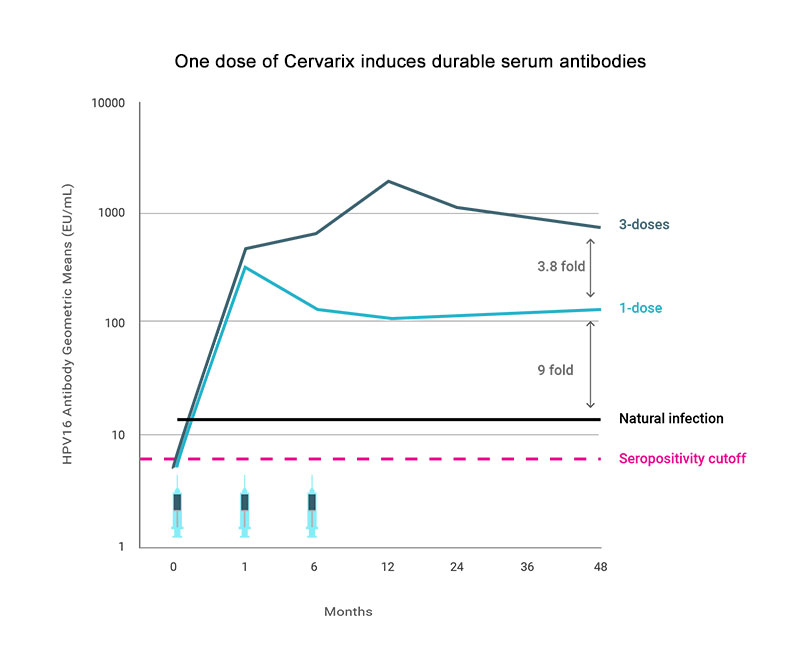
Figure 1. Geometric mean antibody titers (GMT) HPV16 VLP ELISA results by number of vaccine doses from a post-hoc analysis of the Costa Rica HPV vaccine Trial of 18-25 year old women. Natural infection are the GMT levels of seropositive women at enrollment. Adapted from [3].
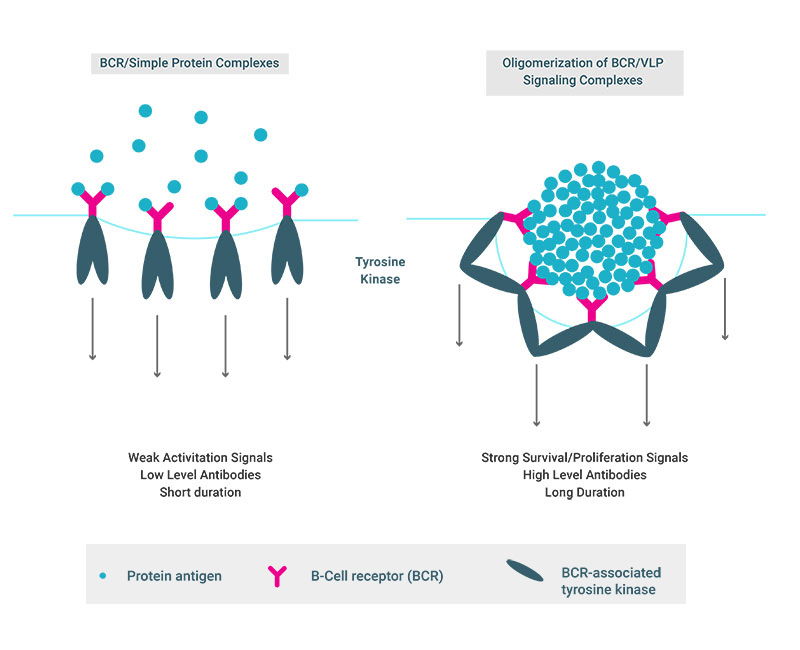
Figure 2. B-Cell Recognition of Dense Repetitive Protein Arrays Promotes the Induction of Exceptionally Potent Antibody Responses

Figure 3. A disrupted epithelium is depicted. The L2 minor capsid protein is cleaved by furin after a heparan sulfate proteoglycan (HSPG) binding-induced conformational change in the capsid, as shown in pink. This results in the exposure of the keratinocyte receptor-binding site on the surface of the virion.
References
1. Kreimer, A. R., et al. 2015. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol, 16, 775-86.10.1016/ s1470-2045(15)00047-9
2. Sankaranarayanan, R., et al. 2016. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol, 17, 67-77.10.1016/s1470 -2045(15)00414-3.
3. Safaeian, M.,et al. 2013. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila), 6, 1242-50.10.1158/1940-6207. capr-13-0203
4. Safaeian, M., et al. 2017. Durability of protection afforded by fewer doses of the HPV16/18 vaccine: the CVT trial. J Natl Cancer Inst. Aug 2017.
5. Gomes, A. C., et al. F. 2017. Harnessing Nanoparticles for Immunomodulation and Vaccines. Vaccines (Basel), 5.10.3390/ vaccines5010006. 6. Schiller, J. T. et al. 2012. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol, 10, 681-92.10.1038/nrmicro2872.
7. Longet, S., et al. 2011. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol, 85, 13253-9.10.1128/ jvi.06093-11
