Will HPV vaccines reduce oropharyngeal cancer burden?
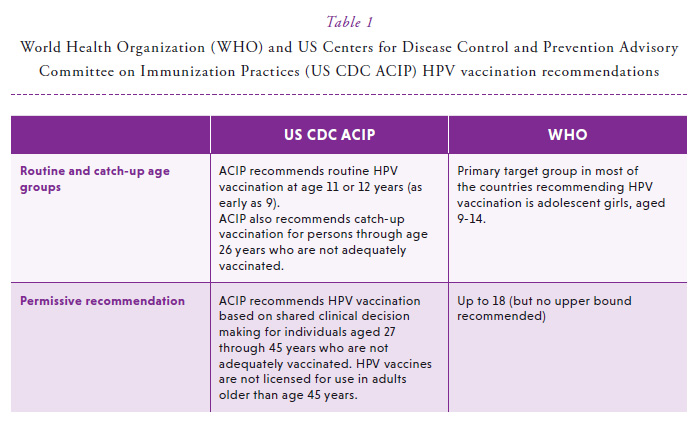
HPV vaccines were licensed and recommended more than a decade ago, in order to reduce individual- and population-prevalence of HPV, a cause of cancer at multiple anatomic sites.1 These vaccines were initially tested and approved for the prevention of cervical precancer. HPV vaccine efficacy was later shown at non-cervical anogenital anatomic sites, including the vulva, vagina, penis and anus.2-4 Currently, the World Health Organization (WHO) recommends vaccination of girls aged 9 to 14 years, the subset of the population with the greatest risk of HPV-induced cancer as well as the likelihood for benefit, and consequently, the most cost-effective strategy.5 The US Centers for Disease Control and Prevention Advisory Committee on Immunization Practices more expansively recommends routine HPV vaccination to females and males aged 9 to 26 and permissive vaccination based on shared clinical-decision making for individuals aged 27 to 45 (Table 1).6 While the vast majority of countries are implementing female-only vaccination programs, 18% of the global HPV vaccine supply is currently utilized by males.5 In 2020, the US FDA did expand the label of the nonavalent HPV vaccine to include prevention of oropharyngeal and other head and neck cancers caused by HPV types targeted by the vaccine. Such approvals could further increase the proportion of countries moving towards gender-neutral HPV vaccination.
Among HPV-naïve individuals, it is highly likely that HPV vaccination, which confers near-complete protection against cervical, vaginal, vulvar, penile and anal HPV infections and precancer, would be similarly efficacious against oral/oropharyngeal HPV infections that cause HPV-positive oropharyngeal cancer. Consistent with this expectation, studies show comparably high efficacy of HPV vaccines in preventing oral HPV. This protection against oral HPV infection arises from vaccine-induced robust systemic neutralizing antibodies, the likely effector mechanism against mucosal HPV infections.7 Indeed, systemic IgG antibodies are the source for salivary IgG antibodies, with high observed correlation in the respective antibody levels.8
The first evaluation of vaccine efficacy (VE) against oral HPV infection was conducted in a randomized clinical trial initially designed to evaluate efficacy of the bivalent HPV vaccine against persistent cervical HPV16/18 infection and precancerous lesions, the Costa Rica HPV Vaccine trial (CVT).9 In this study among young women, a 93% reduction of prevalent oral HPV 16/18 infection was observed in the vaccine arm compared to the control arm approximately 4 years after vaccination.9 Data from the US National Health and Nutrition Examination Survey (NHANES) confirm and expand the CVT observations in a population-representative setting.10 Among young adults (ages 18-33 years), prevalence of vaccine-type oral HPV infections (HPV16/18/6/11) was 88% lower in men and women who self-reported receipt of at least one dose of the HPV vaccine, including a 100% reduction in vaccinated men.10
In addition to direct benefit in vaccinated individuals, emerging evidence also suggests considerable herd protection against vaccine-type oral HPV infections (i.e. indirect benefit among unvaccinated individuals). For example, vaccine-type (HPV16/18/6/11) oral HPV prevalence declined by 37% during 2009-2016 in unvaccinated US men aged 18-59 years, without a commensurate change in the prevalence of non-vaccine oral HPV infections. These results suggest herd protection among unvaccinated men arising from increased vaccine uptake in females.11
To address this, one may consider increasing the age at vaccination—yet, the optimal upper age-limit for catch-up vaccination for the prevention of HPV-positive oropharyngeal cancers remains uncertain. An important question when considering vaccinating older individuals is when the ‘causal’ HPV infection (in other words, the infections likely to cause cancer) is acquired. At the cervix, modeling studies suggest the majority of cervical HPV infections that lead to cervical cancer are acquired by 30 years of age.13 Thus, vaccinating women over the age of 30 years is unlikely to avert a substantial proportion of the cervical cancer burden. Unlike cervical HPV infections, the prevalence/incidence of oral HPV infections does not decline with age.14,15 While the natural history of oral HPV infections and HPV-positive oropharyngeal cancers remain largely unstudied, recent microsimulation modeling suggests that a low proportion of non-cervical cancers are attributable to infections acquired at older ages.16
If a sufficient proportion of causally-relevant oral HPV infections are indeed acquired later in life in men, recommending bodies in countries with high OPC burden may consider expanding to gender-neutral vaccination and increasing the age range of vaccination (although cautiously, to not incentivize older age at vaccination). However, this should only be considered once the current HPV vaccine supply constraints have been addressed.17 Until that time, expanded recommendations should be curtailed in order to prioritize the target population who will receive the most significant benefit, girls aged 9-14 years.5
CONFLICT OF INTERESTSThe authors declare nothing to disclose.
References
1. Arbyn M, Xu L. Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Rev Vaccines 2018;17(12):1085-91. Available at: https://pubmed.ncbi.nlm.nih.gov/30495978/
2. Giuliano AR, Palefsky JM, Goldstone S et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011;364(5):401-11. Available at: https://pubmed.ncbi.nlm.nih.gov/21288094/
3. Muñoz N, Kjaer SK, Sigurdsson K et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010;102(5):325-39. Available at: https://pubmed.ncbi.nlm.nih.gov/20139221/
4. Palefsky JM, Giuliano AR, Goldstone S et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011;365(17):1576-85. Available at: https://pubmed.ncbi.nlm.nih.gov/22029979/
5. World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, October 2019: conclusions and recommendations. Weekly Epidemiologic Record. NOVEMBER 2019, No 47, 2019, 94, 541–560. Available at: https://apps.who.int/iris/handle/10665/329963
6. Meites E, Szilagyi PG, Chesson HW et al. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2019;68(32):698-702. Available at: https://pubmed.ncbi.nlm.nih.gov/31415491/
7. Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol 2012;10(10):681-92. Available at: https://pubmed.ncbi.nlm.nih.gov/22961341/
8. Parker KH, Kemp TJ, Isaacs-Soriano K et al. HPV-specific antibodies at the oral cavity up to 30 months after the start of vaccination with the quadrivalent HPV vaccine among mid-adult aged men. Vaccine 2019;37(21):2864-9. Available at: https://pubmed.ncbi.nlm.nih.gov/31005426/
9. Herrero R, Quint W, Hildesheim A et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 2013;8(7):e68329. Available at: https://pubmed.ncbi.nlm.nih.gov/23873171/
10. Chaturvedi AK, Graubard BI, Broutian T et al. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J Clin Oncol 2017:JCO2017750141. Available at: https://pubmed.ncbi.nlm.nih.gov/29182497/
11. Chaturvedi AK, Graubard BI, Broutian T et al. Prevalence of Oral HPV Infection in Unvaccinated Men and Women in the United States, 2009-2016. JAMA 2019;322(10):977-9. Available at: https://pubmed.ncbi.nlm.nih.gov/31503300/
12. Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J Clin Oncol 2015;33(29):3235-42. Available at: https://pubmed.ncbi.nlm.nih.gov/26351338/
13. Burger EA, Kim JJ, Sy S, Castle PE. Age of Acquiring Causal Human Papillomavirus (HPV) Infections: Leveraging Simulation Models to Explore the Natural History of HPV-induced Cervical Cancer. Clin Infect Dis 2017;65(6):893-9. Available at: https://pubmed.ncbi.nlm.nih.gov/28531261/
14. Gillison ML, Broutian T, Pickard RK et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA 2012;307(7):693-703. Available at: https://pubmed.ncbi.nlm.nih.gov/22282321/
15. Kreimer AR, Pierce Campbell CM, Lin HY et al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet 2013. Available at: https://pubmed.ncbi.nlm.nih.gov/23827089/
16. Laprise JF, Chesson HW, Markowitz LE et al. Effectiveness and Cost-Effectiveness of Human Papillomavirus Vaccination Through Age 45 Years in the United States. Ann Intern Med 2020;172(1):22-29. Available at: https://pubmed.ncbi.nlm.nih.gov/31816629/
17. Kreimer AR et al. Prioritisation of the human papillomavirus vaccine in a time of constrained supply. Lancet Child & Adolescent Health 2020. Vol 4, p 349 to 251. Available from: https://pubmed.ncbi.nlm.nih.gov/32078808/
ARTICLES INCLUDED IN THE HPW SPECIAL ISSUE ON HPV IN OROPHARYNGEAL CANCER:
AR Kreimer, T Waterboer. Screening for HPV-Driven Oropharyngeal Cancer
M Taberna, R Mesia, RL Ferris. Clinical management of HPV-related recurrent/metastatic (R/M) oropharyngeal cancer patients
MJ Windon, EM Rettig. Counseling patients with a diagnosis of human papillomavirus-positive oropharyngeal cancer
S Huang. Oropharyngeal Carcinomas: the UICC/AJCC TNM Staging System, 8th Edition
G D’Souza, A Chaturvedi, E Bigelow. Natural history, from oral HPV infection to HPV-related oropharyngeal cancer


